Cholera Vaccines Unnecessary, Ineffective, Expensive, and Dangerous
Editorial Comment
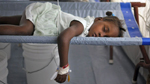
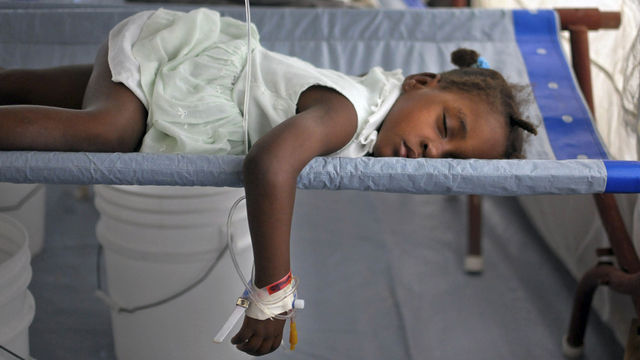
Cholera is a public health problem. Unlike the infectious diseases, such as the flu or the measles, that traditionally require vaccines because they are transmitted in ways (aerosols, casual contact) against which one is defenseless, cholera infections are preventable without vaccines, because cholera is acquired principally from water and foods that have become contaminated with cholera bacteria from feces. Hand-to-mouth transmission is easily avoided with good hygiene, as done by those health workers who handle thousands of cholera patients without ever becoming infected.
Just as cholera was eradicated from 19th century Europe by provision of sanitation and clean drinking water, so it will ultimately be removed from Haiti and the countries in Asia and Africa where cholera is currently endemic. Nevertheless, cholera vaccines are being aggressively promoted for the poor of Haiti. It is possible that cholera vaccination programs attract the worst elements of science because such programs fly in the face of the idea that clean drinking water is a human right.
Haiti Chery is enormously grateful to Dr. Rashid Haider for contributing the first article below. It is one that names names and closely examines the lies that are being promoted by cholera-vaccine merchants. For those readers who want more background on the origins of the vaccines being pushed on Haiti, I also append an older article by Dr. Haider.
Dady Chery, Editor
Haiti Chery
Transparency required in WHO cholera vaccination programme: scientists commercially linked to cholera vaccines control them and manipulate facts
By Rashid Haider
Haiti Chery
Lack of transparency prevails widely in the cholera vaccination programme of the World Health Organization (WHO).
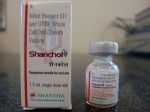
In recent years the WHO has pre-qualified two commercial oral cholera vaccines, Dukoral and Shanchol. Both vaccines contain large amount of killed cholera bacteria, with Dukoral having an additional component known as the recombinant B subunit of cholera toxin (rCTB).
These are “two-dose cholera vaccines” to be taken with a gap of at least two weeks, protection developing one week after the final intake.
Dukoral has been found to offer short term protection, and Shanchol has been found to offer only 45 percent protection after one year of surveillance in a large scale field trial conducted in Kolkata, India.[1]
Neither of these vaccines is suitable for controlling endemic and epidemic cholera.
The Office of Research Integrity (ORI) of the U.S. Government strives to promote responsible conduct of research and urges authors of scientific reports to maintain scientific integrity by
- (i) being accurate and honest,
- (ii) relying upon primary literature and
- (iii) avoiding the publication of selective information. [2]
But the WHO has turned a blind eye to these principles, as evident from its numerous reports that contain false, fabricated, and selected information on the composition, efficiency, and cost of the commercial killed-cholera oral vaccines.
Dukoral
Contrary to the WHO’s propaganda, no Dukoral with rCTB has ever been subjected to a large-scale field trial in Bangladesh.
Sanchol
Shanchol’s poor protective efficacy of 45 percent after one year of surveillance was concealed from the WHO’s position paper on cholera vaccines.[3] Furthermore, the WHO’s claim of Shanchol being a low-cost vaccine in its position paper is without any foundation. [3]
One scientist linked with the WHO’s cholera vaccination program has amassed large sums of money from the trial results of an oral cholera vaccine that had used poor women and children of Bangladesh as experimental animals.
Such questionable activities arise from the fact that the Cholera Vaccine Working Group is dominated by a group of scientists commercially linked to the oral cholera vaccines Dukoral and Shanchol.
Let us consider the composition of the 28 October 2009 Cholera Vaccine Working Group of the WHO that recommended the use of these vaccines against cholera. Of 11 members of the group:
- Five members (John Clemens, Ann-Mari Svennerholm , David Sack, Balakirish Nair, Dipika Sur) were actively associated with Dukoral and Shanchol.[4]
- Five members (Zulfikar Bhutta, Anita Zaidi, Mitchell Weiss, Duncan Steele, Myriam Henekens) could be considered passive bystanders, since they had hardly made any contributions in the field of cholera, and their names appeared only sporadically in minor publications.
- Only one member (James Kaper) was a reputable scientist working with live oral cholera vaccines. Unfortunately, his vaccine (CVD 103-HgR) had not worked when field tested in Indonesia in 1992.
There were a few other participants in that Working Group meeting. With one exception (Eric Mintz from the Centers for Disease Control and Prevention, USA), most had made little contribution to the field of cholera.
How were the members of the Working Group selected?
One can demand transparency in the selection of the WHO’s cholera vaccine experts.
Like the WHO’s swine flu experts, the vast majority of the WHO’s cholera experts are commercially linked with the cholera vaccines. They push their agenda through the WHO by manipulating information and advocating the use of ineffective but commercially profitable vaccines such as Dukoral and Shanchol.
The decision of the WHO to use cholera vaccines should be carried out by independent-minded cholera scientists, and those commercially linked with cholera vaccines should be barred from the WHO’s Cholera Vaccine Working Group.
There should be no compromise on the question of scientific honesty, as the WHO must adhere to the universally accepted guidelines of scientific integrity put forward by the Office of Research Integrity.
The Bill and Melinda Gates Foundation, which financially supports the field trials of Shanchol, should see to it that the principles of the Office of Research Integrity are strictly obeyed by the scientists associated with Shanchol.
References
1. Lancet 2009; 374:1694
2. http://ori.hhs.gov/education/products/plagiarism/1.shtml
3. Weekly Epidemiological Report, No 13, 2010, 85, 117-128
4. www.who.int/immunization /SAGE_cholera_overview_slides_10-28-09_Jon_Abramson.pdf
Source: Haiti Chery
Racketeering in WHO cholera vaccination programme: public funds channeled into private pockets
By Rashid Haider
News from Bangladesh
In November 2008, Dr. Margaret Chan, the Director-General of the World Health Organization (WHO), appealed to all governments for financial helpwhen the world was going through a deep financial and economic crisis.[1] But has Dr. Chan kept her house in order? Allegations supported by documents unequivocally prove the contrary.
Cholera is a social disease mediated by bacteria and is easy to cure. Cholera prevails in several countries of Asia, Africa, and Latin America, taking a toll of human lives.
The mode of cholera transmission is by the faecal-oral route through inadequately treated water, food, or by contamination of utensils and other household items.
The WHO maintains several programmes: the vaccination programme against the diarrhoeal disease cholera being one of them. The WHO has not produced a vaccine yet that offers a long-term protection against cholera.
A critical look into the activities of the cholera vaccination programme of the WHO will unravel the workings of evil forces that are operating under the umbrella of WHO. A brief account of such activities is given below.
A. WHO squanders away millions of dollars to develop vaccines for rich tourists under the guise of helping the poor
A club comprising a few scientists from Sweden (Jan Holmgren and wife Ann-Mari Svennerholm, Gothenburg University), South Korea (John Clemens, the International Vaccine Institute) and the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) controls the cholera vaccination programme of WHO. To make money out of human misery is the motto of the club members who are using WHO as the vehicle to accomplish their goals.
In 1985 these scientists, with the aid of WHO, carried out an oral cholera vaccine trial involving 90 000 women and children of Bangladesh. The vaccine, comprising large amounts of killed cholera bacteria and the B subunit of cholera toxin (CTB), is very expensive to produce. The United States Agency for International Development (USAID) alone contributed U.S. $2.3 million during 1985-86 to the Bangladesh vaccine trial.[2]
Production of a tourist vaccine was not the objective of the trial.
The idea of using CTB in a vaccine was originally conceived by pioneering cholera scientist Professor R. A. Finkelstein of the University of Missouri, Columbia, USA. Because of the vaccine’s exorbitant cost, Prof. Finkelstein correctly felt that the poor people of the world would be unable to afford it. WHO was aware of Prof. Finkelstein’s opinion, which was documented in 1983 in a reputable journal dealing with the standardization of vaccines.[3] But Dr. Holmgren, the man behind the vaccine trial, was also a member of the Diarrheal Committee of WHO. He manipulated WHO to overrule any objections.
The performance of this highly expensive vaccine was dismal. It did not offer protection long enough to meet the requirements for mass immunization. The vaccine was practically ineffective in children, who were the targeted population in heavily endemic areas such as Bangladesh.
- After monitoring for one year, it was found that more children in Bangladesh who took the vaccine had died of cholera than those who had received a placebo.[4]
- The vaccine actually increased the risk of cholera in children in the third year.[5, 6]
Thus, the WHO had squandered away millions of dollars on the development of a vaccine that was never meant for the poor in the first place.
The oral cholera vaccine does not meet the requirements of the Food and Drug Administration (FDA) and is unavailable in the USA. But WHO, overruling the FDA objections, approved this oral cholera vaccine in 1999.[7]
The real objective of the Swedish scientists was to get rich quick by developing a vaccine for tourists from rich nations who might require short-term protection at any cost. But these scientists had obtained the funds for their research and development from USAID and WHO using the name of the poor cholera sufferers of Bangladesh.
The Government of Sweden has used the results of the Bangladeshi trial to market this oral cholera vaccine.[8] Since the mid 1990s, The Government of Sweden had been making huge profits by selling this vaccine to soldiers and tourists of rich nations through its vaccine producing laboratory SBL-Vaccin AB (currently owned by a Dutch private company Crucell BV, Archimedesweg 4, 2333 CN Leiden, The Netherlands). The vaccine is being sold under the trade name Dukoral at the enormous cost of 450 Swedish Crowns (approximately US $65 to $75). In 2005 alone, SBL Vaccin AB generated approximately EURO 25.2 (US $36) million in revenues[9], thanks to the help that WHO offered to a private vaccine company such as SBL Vaccin AB of Sweden.
The needs of the poor, who due to social conditions suffer from diseases like cholera, are ignored by WHO.
B. Accumulation of massive wealth by Dr. Holmgren through the USAID-WHO sponsored cholera vaccine trial in Bangladesh
Dr. Holmgren has been working as an expert in programmes on diarrhoea and vaccines of WHO for several years, beginning from the 1980s. He and his wife Ann-Mari Svennerholm claim to be the inventors of the Dukoral vaccine.[8]
Dr. Holmgren illegally obtained a patent on CTB for the Dukoral vaccine in a number of countries, including Sweden and USA (The US Patent # 5268276 is dated Dec 7, 1993). He concealed from his patent application the information on the financial support he had received from WHO for his work on CTB.[10] Moreover, he applied as a private person, concealing his place of employment. He draws large sums of money in royalties from the sales of the vaccine Dukoral with CTB.
On 29 June 1998 Dr. Holmgren obtained for his CTB a cash payment of Swedish crowns 25.6 million (approx. U.S. $3 to $4 million) and an agreement on a large number of future shares from the owner of the Swedish company SBL Vaccin AB.[11] But the vaccine Dukoral was possible only because of the trial that had been performed on 90,000 Bangladeshi women and children. This was not mentioned in the patent application aor the brochure produced by SBL Vaccin AB.[8] WHO and a number of governments such as those of the USA, Japan, Canada and Bangladesh had funded this vaccine trial.[12] But the Swedish vaccine producers (SBL-Vaccin AB) denied this fact and instead stated falsely that SBL had financed the development and clinical testing without external assistance.[11]
The marketing of the vaccine Dukoral is illegal since it involves cheating the financial donors such as WHO and several governments (USA, Canada, Japan and Bangladesh) and 90 000 Bangladeshi trial participants.
Thus there is a racketeering in which the Swedish government, a private organisation (SBL-Vaccin AB), two Swedish governmental scientists (Dr. Holmgren and wife Ann-Mari Svennerholm), and their associates (Drs. David Sack and John Clemens) have violated the human rights of the poor people of Bangladesh. They have used them as substitutes for laboratory animals to test highly expensive biological materials with a view to make profits.
Accusations of ethical violations, as defined by the Declaration of Helsinki, in the vaccine trial were raised both inside Bangladesh and Sweden.[13] The matter was even debated in the Swedish Parliament.[14]
Dr. Holmgren, Dr. Ann-Mari Svennerholm, and SBL-Vaccin AB must return all the ill-gotten money they have made not only to the donors (WHO, USAID, and other governments), but also to the 90 000 poor women and children of Bangladesh who can be easily identified through the database of the ICDDR,B.
C. WHO’s false propaganda to promote the tourist oral cholera vaccine
Catalogue of lies and factual distortions
For a number of years the Weekly Epidemiological Record (WER), a publication of the World Health Organization (WHO), has been publishing false and fabricated materials related to the oral cholera vaccine Dukoral that is predominantly used by tourists. WER in its July 30, 2004 issue published the following:
One vaccine consists of killed whole-cell V. cholerae O1 with purified recombinant B-subunit of cholera toxoid (WC/rBS). Field trials in Bangladesh, Peru and Sweden have shown that this vaccine is safe and confers 85-90% protection during 6 months in all age groups after administration of two doses, one week apart. In Bangladesh, protection declined rapidly after 6 months in young children, but was still about 60% in older children and adults after two years.[15]
The above statements are full of lies. These are as follows:
1. The statement that 2 doses of the WC-BS vaccine were given in Bangladesh is a lie.
It was a 3 spaced dose vaccine that was orally administered to 21,141 individuals in 1985 in Bangladesh, a cholera endemic country.[16]
2. It has been mentioned that the WC/rBS vaccine that contained the B subunit of cholera toxin obtained by recombinant DNA technology was used in a field trial in Bangladesh. That is another lie.
The B subunit of cholera toxin (BS) used in Bangladesh in 1985 was not obtained by recombinant DNA technology. It was derived from cholera toxin (CT) using chemical methods. The WC/rBS came into use only after 1992.
The results from BS preparations from the two different sources are not necessarily the same. BS obtained by chemical purification may contain traces of CT, a very powerful adjuvant [activator of the immune response] [17] that may remain undetected in the quality control assay but be sufficient to exert some biological functions [inflammation, irritation]. On the other hand, the rBS constructed by recombinant technology involves different methods of purification that does not encounter CT. It is possible that the substitution of BS by rBS might have weakened the combination vaccine.
The Dukoral that WHO is promoting is a weaker version of the vaccine tested in Bangladesh in 1985.
When Dukoral was tested in Peru in the 1990s in a large scale field trial conducted by scientists not associated with the WHO-linked Swedish scientists, the vaccine produced dismal results.[18] These results highlighted the fact that all vaccine trials should be monitored by independent observers without conflict of interest. However, Dr. Claire Lise Chaignat, Director of the Cholera Control Programme of WHO, concealed these findings and deceived readers by making false claims in a scientific journal about the efficacy of Dukoral.[19] She wrote lies similar to those published earlier in WER.[15,19]
3. The fictitious field trial of the oral cholera vaccine of WHO in Sweden
This statement that the vaccine was subjected to a field trial in Sweden has been appearing in WER for years. The claim by WHO that the vaccine had undergone a field trial in Sweden is false and utterly misleading.[15] Sweden is not a cholera endemic country. No controlled field trial was carried out in Sweden with this vaccine. Testing for immunogenicity of the vaccine on a handful of healthy volunteers in a non-endemic country without any later challenge study with live Vibrio cholerae O1 does not constitute the field trial of a vaccine.
This leads one to ask for the fundamental definition of a field trial. When did the field trial in Sweden take place? How many people were involved in the trial? What was the design of the evaluation process? Was it a randomized, double-blind, controlled-trial? One wonders!
D. The oral cholera vaccine fails to meet the challenges of the cholera epidemic
Recently WHO acknowledged the limitations of Dukoral that make it unsuitable as a cholera control measure in epidemics.[20]
- Since Dukoral is a multiple dose vaccine, administered with an interval of 10-14 days, it is of little use once a cholera outbreak has started.
- A large volume of safe water (75-150 ml) is needed for the administration of each dose. The vaccine has a strict requirement of cold chain and large volume of clean water.
- The vaccine cannot be given to children under two years of age.
- The short term protective efficacy of the vaccine, poor protection in younger children, reduced protection against one type (El Tor) of cholera and very high cost make it unsuitable for controlling endemic and epidemic cholera.
That is why Dukoral was not used in recent cholera outbreaks in Iraq and Zimbabwe.[20,21]
E. Scientists with links to Vietnam and India are currently lobbying to obtain approval fromWHO for marketing another ineffective oral cholera vaccine for global use
Scientists (Drs. Jan Holmgren and John Clemens) deeply involved in the Cholera Control Programme of WHO have been working for several years in Vietnam. They wanted to market an oral cholera vaccine comprising large amounts of killed cholera bacteria (about 175 billion bacteria). The Vietnamese drug agency is not internationally recognized, and so they decided to exploit the Indian drug agency, which is globally accepted. They are now manufacturing the Vietnamese oral cholera vaccine in India (Shantha Biotechnics, Hyderabad) and have recently marketed it while concealing vital information concerning the composition and protective efficacy of the vaccine.
The vaccine suffers from a number of drawbacks.
1. Poor protection against cholera
The company and its scientists claimed that the vaccine offered 70% protection over two years when tested on several thousand slum dwellers of Kolkata.[22] But they have concealed the fact that the vaccine offered poor protection (40-45%) after one year.
If the vaccine protected only 40-45% during the first year, how was it scientifically possible that its protective efficacy increased up to 70% during the second year?
Is the information coming out of the trial reliable? One wonders. As the vaccine demonstrated poor efficacy soon after its administration, it cannot be regarded as an effective vaccine to control cholera.
2. Is the vaccine cheap?
Scientists associated with this vaccine have launched a media campaign claiming that the vaccine is very cheap.[22] A few years ago Director General Dr. Hiroshi Nakajima of WHO mentioned that the cost of a vaccine should not exceed one dollar per dose, otherwise it would be out of reach for most of the world. But as reported widely in the Indian press[23], the two-dose Indian oral cholera vaccine will be sold by the vaccine producer (Shantha Biotechnics) at the cost of the Indian Rupees 600 (US $12). It is absurd that a vaccine that costs US $12 in India should be regarded cheap.
3. Vaccine should be mercury free
Contrary to the recommendation of the WHO Expert Committee on Biological Standardization[24], the vaccine contained the toxic mercury-based preservative thiomersal. Questions have particularly arisen around a possible connection between thiomersal and autism. Hence, thiomersal as a vaccine-preservative is not in use in many developed countries including Denmark, Sweden and several states of USA[25, 26, 27].
The Indian vaccine company has not disclosed the amount of mercury present per dose of the vaccine. Because of the controversies associated with the use of thiomersal, the vaccine manufacturer should have used other, non-harmful, preservatives.
It is worthwhile to point out that thiomersal is not used in vaccines in the country (Sweden) of Dr. Holmgren, a prime mover of the Vietnamese oral cholera vaccine.[26] But he has no problem in using the mercury containing vaccines in other countries (Vietnam and India), clearly providing an example of practicing a double standard.
Drs. Jan Holmgren and John Clemens are currently lobbying inside WHO to get its seal of approval for this ineffective oral cholera vaccine in order to capture the global cholera vaccine market.
F. Concluding remarks
While cholera persists in some parts of the world, opportunist elements operating inside WHO are trying to promote ineffective vaccines with a view to make money out of human misery. They did this earlier in the 1980s for a cholera vaccine now sold as Dukoral to the tourists of rich nations. Now they are lobbying for WHO approval of an ineffective oral cholera vaccine that offers poor protection soon after its intake.
Audit requested
As the US government is the major funder of WHO, the US must audit the financial activities of WHO so that dishonest persons currently operating inside its cholera vaccination programme cease to function.
The marketing of Dukoral, the tourist oral cholera vaccine, could not have been accomplished without funding from several governments (USA, Japan, Canada and Bangladesh) and use in the vaccine trial in Bangladesh in 1985. Therefore Dr. Jan Holmgren, Dr. Ann-Mari Svennerholm and the vaccine company SBL-Vaccin AB (present owner being Crucell NV of the Netherlands) should return the funds to these governments.
FDA approval of vaccines essential
At present evil forces are lobbying to obtain WHO approval for the ineffective Vietnamese oral cholera vaccine manufactured in India. The US government should not support WHO approval for cholera vaccines that are not recommended by FDA.
Scientific integrity of WHO should be investigated
WHO is obliged to maintain scientific accuracy and honesty in providing information to the public. As documented in this report, publication of false and misleading information has become a routine practice of the WHO cholera vaccination programme. An investigation into such WHO practices should be launched. Persons associated with the dissemination of false information in WER and in scientific journals should be brought to justice.
References
1. Chan M. Impact of the global financial and economic crisis on health. World Health Organization (12 November 2008).
2. The International Centre for Diarrhoeal Disease Research, Bangladesh. Annual Report 1985; page 53.
3. Svennerholm AM, Jertborn M, Gothefors L, Karim A, Dack DA, Holmgren J. Current status of an oral B subunit whole cell cholera vaccine. Dev Biol Stand 1983; 53:73-79.
4. Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Khan MU, Kay BA, Huda N, Khan MR, et al. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60-9.
5. Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270-3.
6. Finkelstein RA. Why do we not yet have a suitable vaccine against cholera? Adv Exp Med Biol. 1995;371B:1633-40. Review.
7. Cholera vaccines: a new public health tool? Report of a meeting, Geneva, 10-11 December 2002, Geneva, World Health Organization, 2004 (CDS/CPE/ZFK/2004.5)
8. DUKORAL: Resevcciner fran SBL Vaccin, 105 21 Stockholm, Sweden. 1996
9. Annual Report Crucell BV (The Netherlands) 2008 http://investors.crucell.com/C/132631/PR/200611/1089281_5_5.html
10. Sanchez J, Holmgren J. 1989. Recombinant system for over expression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proceedings of the National Academy of Sciences U S A. 86(2):481-5.
11. The Internet press release, Active Biotech/SBL Vaccin AB, June 28, 1998.
12. Clemens JD, Harris JR, Khan MR, Ali M, Younus M, Khan MU et al., Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. The Lancet 1988 Jun 18: 1375-79.
13. Mazhar F. 1996. Women and children of Bangladesh as experimental animals. Dhaka (ISBN No. 984-467-050-0).
14. Riksdagsdebat (The parliamentary Debate, Sweden) 1987. March 26:178 (Stockholm, Sweden).
15. Cholera, 2003. Weekly Epidemiological Record, No 31, 2004, 79, 281-288.
16. Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, Kay BA, Khan MU, Yunus M, Atkinson W, et al. Lancet. 1986 Jul 19;2(8499):124-7.
17. Northrup RS, Fauci AS. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis. 1972 Jun;125(6):672-3.
18. Taylor DN, Cardenas V, Sanchez JL, Begue RE, Gilman R, Bautista C, Perez J, Puga R, Gaillour A, Meza R, Echeverria P, Sadoff J. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J Infect Dis. 2000 May;181(5):1667-73.
19. Girard MP, Steele D, Chaignat CL, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine. 2006 Apr 5;24(15):2732-50.
20. WHO position paper on cholera vaccine use in Iraq, 05 October 2007
21. Koenig R. International groups battle cholera in Zimbabwe. Science 2009, 323:860-1.
22. The Times of India, Oral Cholera vaccine may soon be used in India, 11 April 2009
23. Business Daily from The Hindu group of publications, Oral cholera vaccine Shanchol from Shantha for India, 27 April 2009.
24. WHO Expert Committee on Biological Standardization 52nd Report, page 137, 2004.
25. Doctors group fights thiomersal ban. NewsMax.com Wires June 15, 2006.
26. Response to the HEAL/HCWH questionnaire to EU by member states, Mats Welin, Medical Products Agency.
27. Danish Medicines Agency. Indikation 14 July 2004.
Source: News from Bangladesh



Comments
Cholera Vaccines Unnecessary, Ineffective, Expensive, and Dangerous — No Comments